
What is Reverse Osmosis
Reverse osmosis is the process in which the passage of solvent molecules from the more concentrated solution to the less concentrated solution is forced, and is obtained by applying a pressure greater than the osmotic pressure to the more concentrated solution. In practice, reverse osmosis is achieved with a membrane that holds the solute on one side, preventing its passage and allowing the pure solvent to be obtained on the other. This phenomenon is not spontaneous and requires the completion of mechanical work equal to that necessary to cancel the effect of osmotic pressure.
This process represents the finest water filtration technique, as it does not simply consist in overcoming a physical obstacle to the passage of molecules (determined by the size of the pores), but exploits the different chemical affinity with the membrane, allowing in fact the passage of hydrophilic (or water-like) molecules, i.e. chemically similar to water (e.g. short-chain alcohols).
From the plant engineering point of view, the method exploits the principle of cross-flow filtration, as well as for other separation techniques using membranes such as microfiltration, ultrafiltration and nanofiltration.
Reverse osmosis is used in water treatment , both for desalination and for removal of traces of phosphates, calcium and heavy metals, as well as pesticides, radioactive materials and most of the polluting molecules .
Reverse osmosis on membrane
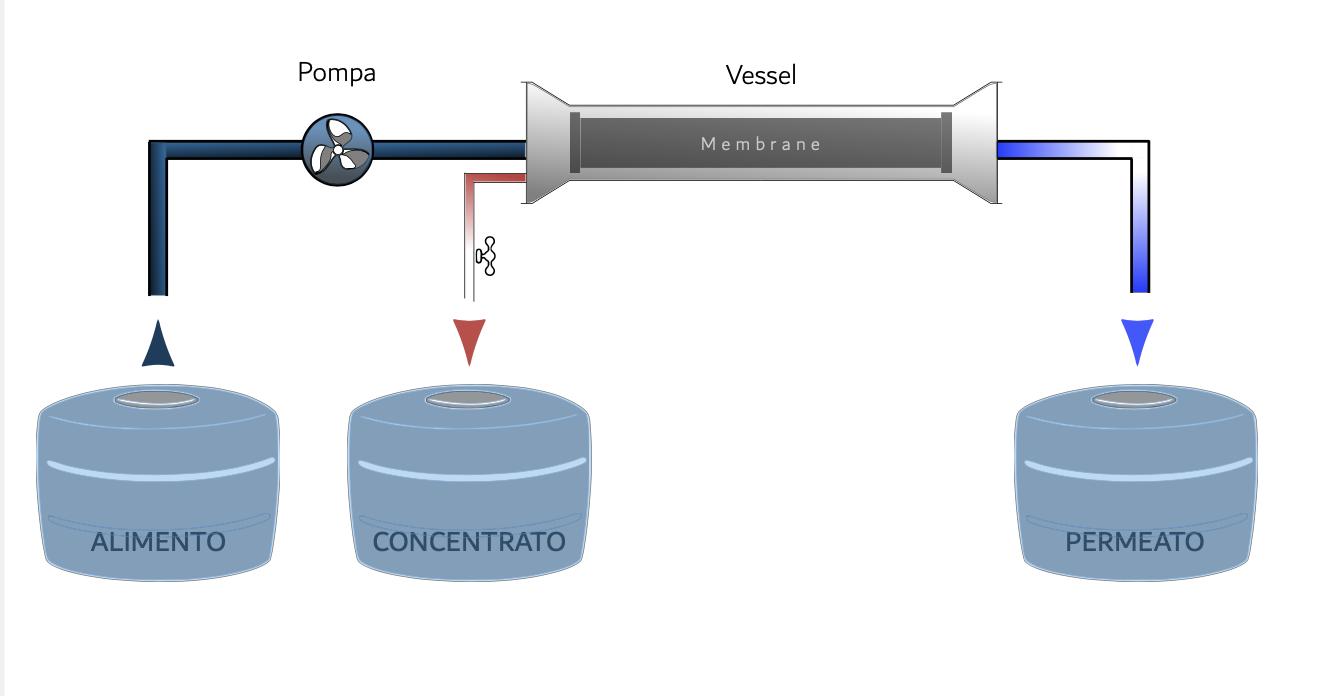
It is a membrane process, which allows you to remove almost all of the substances present in it from the water. The feed water is pressurized by a pump that exerts a pressure higher than the osmotic one, obtaining two flows: the permeate, poor in salts and the concentrate with a high salt concentration, due to the accumulation of all the salts that do not have crossed the membrane.
The membranes used
In the reverse osmosis process, composite membranes are used, consisting of thin films (TFC or TFM, Thin Film Composite Membrane). These membranes are semi-permeable and manufactured mainly for use in water purification or desalination systems. They also have uses in chemical applications such as batteries and fuel cells. Basically, a TFC material is a molecular sieve made with a film of two or more layered materials.
The membranes used in osmosis are generally made of polyamide, chosen mainly for its permeability to water and its impermeability to various dissolved impurities, including saline ions and other small molecules that cannot be filtered. Another example of a semipermeable membrane is that used in dialysis.
Fields of use Reverse Osmosis
- Organoleptic improvement of drinking water for domestic use
- Potabilization of surface, groundwater and marine waters
- Water treatment of reservoirs
- Demineralized water for the electronics and glass electroplating industries
- Demineralized water to feed the steam boiler
- Hospitals and analysis laboratories
- Pharmaceutical and cosmetic industry
- Environment (reuse)
Criticality of the Reverse Osmosis process
Scaling
Scaling consists in the isolation of suspended inorganic particles, such as calcium carbonate, barium sulphate and iron residues.
Fouling
Fouling consists in the isolation of organic, colloidal and suspended particles. Bacteria and other microorganisms that decompose these particles will generate substrates. As a result, they will grow and develop further.
Advantages of a reverse osmosis system
- Water saving
- Energy saving
- Certified materials
- Controlled construction process
- Proven operation